Choosing the Right Nylon: Exploring the Difference Nylon 6 Vs. Nylon 66
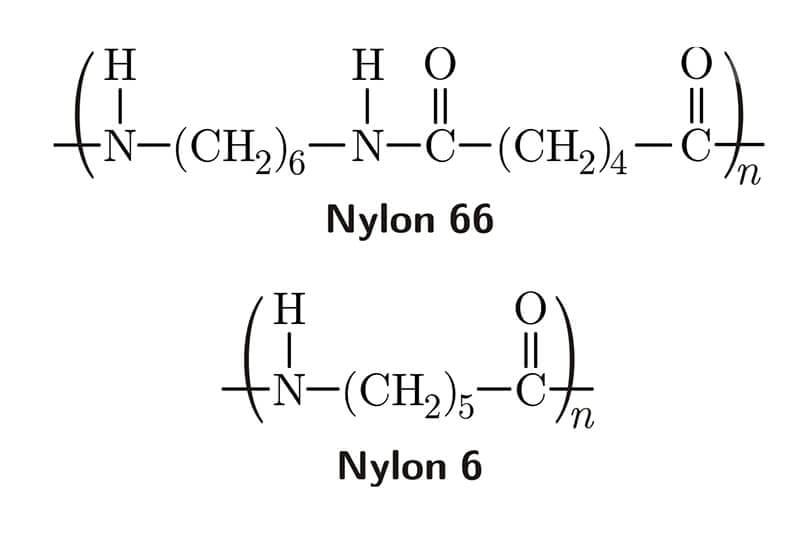
Nylon 6 is made from caprolactam, which has six carbon atoms. Nylon 6/6 is made from hexamethylene diamine, which has six carbon atoms, and adipic acid, which has six carbon atoms.
Both Nylon 6 and Nylon 66 are made of polyamide. A polyamide is a long chain of amide bonds (-CO-NH-) that can be made in a lab or found in nature. Polyamides like Nylon 6 and Nylon 66 are made in a lab. Nylon 6 is not a condensation polymer. Instead, it is a semicrystalline polyamide. Polyamide also comes in the form of Nylon 66. The most significant difference between Nylon 6 and Nylon 66 is that Nylon 6 is made through a process called “ring opening polymerization, while nylon 66 is made through a process called “condensation polymerization.
Nylon 6: Everything you need to know
Nylon 6 has the same strength, stiffness, and toughness as the rest of the nylon family. It does an excellent job of keeping noise out and heat in. Some people call it PA6.
It slides with little friction, doesn’t tire, and wears little over time. It is also easy to work with and can quickly be made into its final shape. It also has a shiny surface.
Common characteristics of Nylon 6
Common Applications of Nylon 6
Advantages of Nylon 6
Disadvantages of Nylon 6
Nylon 66: Everything you need to know
Nylon 66, or more specifically, nylon 66, is a type of polyamide that combines a diamine and a dicarboxylic acid. Hexamethylenediamine and adipic acid are the monomers used to make nylon 66. Both substances have 6 carbon atoms, so their polymer is called nylon 66 or PA66.
Common Characteristics of Nylon 66
Common Applications of Nylon 66
Advantages of Nylon 66
Nylon 66 is the nylon group that is used the most:
Disadvantages of Nylon 66
Comparison Between Nylon 6 Vs. Nylon 66
| Type | Nylon 6 | Nylon 66 |
|---|---|---|
| Short description | Polycaprolactam, also known as Nylon 6, is a type of polymer called semicrystalline polyamide. | The ring-opening polymerization of caprolactam makes nylon 6. Because caprolactam has six carbons, it is called Nylon 6. |
| What ingredients were used to make this | Nylon is used to make rugs, ropes, seat belts, parachutes, industrial cords, hoses, clothing, and hosiery. | Nylon 66 is made of two parts called monomers. hexamethylenediamine, and adipic acid, which each have 6 carbon atoms. This is how Nylon 66 gets its name. |
| applications | Nylon makes rugs, ropes, seat belts, parachutes, and industrial cords. It is also used to make hose, clothing, and hosiery. | Nylon 66 is often used when great mechanical strength, rigidity, good stability at high temperatures, and chemical resistance are needed. |
| Characteristics | Nylon 6 fibers are strong and have a high tensile strength, flexibility, and shine. | The repeat unit of nylon 6,6 has a molecular weight of 226.32 g/mol and a solid density of 1.24 g/(cm3). |
| Pros | Nylon 6 cannot break down easily and has high tensile and impact strength and the ability to be machined and stretched. | Nylon 6:6 has a high tensile strength and lasts long, making it perfect for rugged uses. |
| Cons | The lousy thing about nylon 6 is its low melting point. | No self-extinguishing |
Final Words
The basic material of Nylon 6 and Nylon 66 is polyamide, but their properties are different. Some plastic parts can be made of Nylon 6 and Nylon 66, but most plastic parts can’t.
If you are unfamiliar with Nylon 6 and Nylon 66 and need a trustworthy supplier, don’t hesitate to contact UVTECO, a leading supplier of plastic engineering and machining services.
Related Blogs

Looking for a trustworthy Supplier
Need a Trustworthy Supplier of Plastic, Foam, Sponge, Rubber, Metal, and Machining Solution. Click the Button, We Will Be In Touch With You As Quickly As Possible.